When hives won’t go away - not for weeks, not for months, not for years - it stops being a nuisance. It becomes a prison. For people with chronic spontaneous urticaria (CSU), the skin flares without warning, turning red, swollen, and itchy. Some days, the swelling creeps into the lips or throat. Sleep disappears. Work suffers. Social life shrinks. And if you’ve tried the standard antihistamines and nothing helped? You’re not alone. About 60% of CSU patients don’t get real relief from first-line drugs. That’s where second-line treatments come in - and what’s available now is nothing like it was five years ago.
Why First-Line Treatments Often Fail
Most doctors start with second-generation antihistamines: cetirizine, loratadine, fexofenadine. They’re safe, cheap, and non-drowsy. But here’s the hard truth: only about 40% of people with CSU get at least half their symptoms under control with these. Even when you double or quadruple the dose - which some doctors do - you still only help another 10-15%. That leaves most patients stuck in a cycle of flare-ups and frustration. The reason? CSU isn’t just an allergy. In about half of cases, it’s autoimmune. Your own immune system attacks your skin cells, releasing histamine and other chemicals that cause hives. Antihistamines block histamine, but they can’t stop the immune system from keeping the attack going. That’s why you need treatments that go deeper.Omalizumab: The Longstanding Second-Line Standard
For over a decade, omalizumab is a monoclonal antibody that binds to free IgE, preventing it from triggering mast cells to release histamine. Also known as Xolair, it was the first biologic approved for CSU in 2014. It’s given as a monthly injection under the skin. Most patients see improvement within 4 to 8 weeks. About 30-70% get a meaningful reduction in hives and swelling. But here’s what no one tells you upfront: about 70% of people on omalizumab still have some symptoms. Complete control? That’s rare. And if you have IgG-mediated autoimmune urticaria - which affects at least 30% of CSU patients - omalizumab often doesn’t work at all. It targets IgE, not IgG. So if your body is attacking itself with IgG antibodies, this drug won’t touch the root cause. Still, omalizumab remains the most widely used second-line option. It’s covered by most insurance plans, has a well-known safety profile, and works reliably for many. But it’s not the end of the road anymore.Remibrutinib: The Oral Game-Changer
Enter remibrutinib is a Bruton tyrosine kinase (BTK) inhibitor that blocks signals in mast cells and autoreactive B cells, reducing both histamine release and autoantibody production. In two large phase 3 trials - REMIX-1 and REMIX-2 - involving over 900 adults with CSU who didn’t respond to antihistamines, remibrutinib gave a complete response (no hives, no swelling, no itching) in 28-32% of patients after 24 weeks. That’s comparable to omalizumab’s best numbers. The big difference? It’s a pill. Once a day. No needles. No clinic visits. For people tired of monthly injections, this is huge. Adherence skyrockets when treatment fits into your morning coffee routine instead of your schedule. It also works differently. While omalizumab only targets IgE, remibrutinib hits two key problems at once: it stops mast cells from blowing up and reduces the production of those harmful IgG autoantibodies. That’s why early data suggests it may be more effective for autoimmune CSU - the group that doesn’t respond well to omalizumab. It’s not approved yet as of early 2026, but regulatory reviews are underway. If it gets the green light, it could become the new go-to second-line treatment - especially for younger patients, working parents, or anyone who hates needles.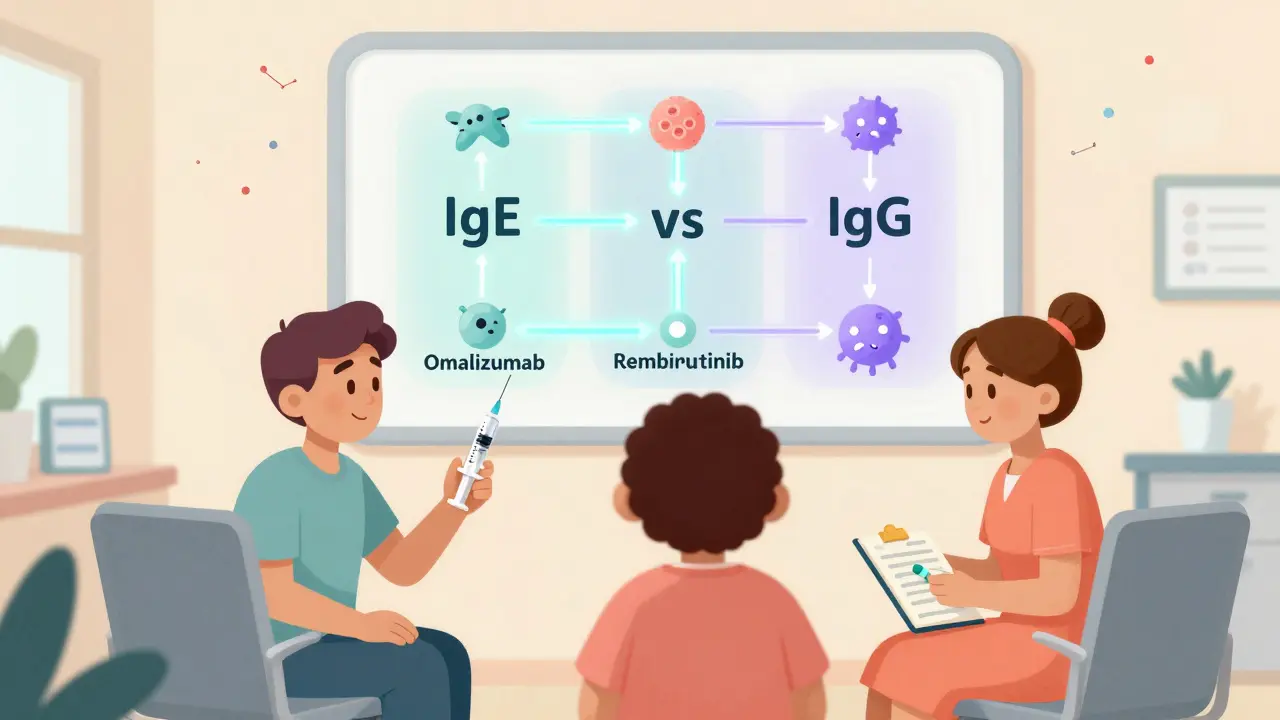
Dupilumab: A New Contender from a Familiar Drug
You’ve probably heard of dupilumab is an anti-IL-4Rα antibody that blocks key inflammatory signals involved in allergic and autoimmune skin reactions. Also known as Dupixent, it’s already approved for eczema and asthma. Now, it’s showing serious promise for CSU. In phase 3 trials, 30-31% of patients achieved complete symptom control at week 24 - slightly better than omalizumab’s average. Dupilumab works by calming down the immune system’s Th2 pathway, which is overactive in many autoimmune skin conditions. It doesn’t just block histamine - it reduces the whole inflammatory cascade behind the hives. The catch? It’s not officially approved for CSU yet. Doctors can prescribe it off-label, but insurance rarely covers it for this use. Still, many dermatologists and allergists are starting to use it for patients who failed omalizumab - especially those with overlapping eczema or asthma. It’s given as a subcutaneous injection every two weeks, so it’s less convenient than a pill but easier than monthly shots.Why Some Drugs Failed - and What That Means for You
Not every new drug made it. fenebrutinib was another BTK inhibitor that looked promising in early trials. But in 2023, its development for CSU was stopped because it caused elevated liver enzymes in some patients - a sign of potential liver damage. That’s a harsh reminder: just because a drug works doesn’t mean it’s safe long-term. This isn’t just a pharmaceutical setback. It’s a warning. The field is moving fast, but safety still comes first. When new options appear, ask your doctor: What’s the long-term data? What are the real risks? Don’t assume “new” means “better.”Cyclosporine: The Old Workhorse with a Heavy Price
If you’ve tried everything else and still have hives, your doctor might mention cyclosporine is an immunosuppressant that reduces T-cell activity and autoantibody production, often used off-label for severe autoimmune CSU. It works. In 54-73% of patients, it clears hives - especially those with autoimmune CSU who didn’t respond to omalizumab. But here’s the catch: it’s not gentle. It can raise blood pressure, damage kidneys, and increase infection risk. Most doctors only use it for short bursts - 3 to 6 months - and monitor blood tests closely. It’s not for long-term use unless you have no other options. Still, for some, it’s the difference between living and surviving. If you’ve been told “there’s nothing else,” cyclosporine might be that last lifeline - but only under careful supervision.
What’s Next? Personalized Treatment Is Coming
The future of CSU treatment isn’t one-size-fits-all. Experts now believe we need to know why your hives are happening. Are your IgE antibodies acting up? Or is it IgG? Are your mast cells overactive? Or are your B cells making bad antibodies? Blood tests to detect autoantibodies are becoming more common in specialist clinics. If you test positive for IgG-mediated CSU, you’re more likely to respond to remibrutinib or cyclosporine than to omalizumab. If you have high Th2 inflammation, dupilumab might be your best bet. Within the next 3-5 years, we’ll likely see treatment decisions based on these subtypes - not just trial and error. That means fewer months of suffering, fewer ineffective drugs, and more targeted relief.What Should You Do Now?
If you’ve been on antihistamines for 6+ weeks and still have hives:- Ask your doctor if you’ve been tested for autoimmune markers (like the autologous serum skin test or basophil activation test).
- Don’t assume omalizumab is your only option - ask about remibrutinib (when available) and dupilumab.
- If you’re on high-dose antihistamines, you’re probably not getting more benefit - it’s time to move on.
- Keep a symptom diary: note when hives flare, what you ate, stress levels, sleep, and menstrual cycle (for women). Patterns matter.
- Find a specialist - an allergist or dermatologist who treats CSU regularly. General practitioners rarely have the depth of experience needed.
Real Talk: It’s Not Just About Hives
People with CSU don’t just have skin problems. They have anxiety, depression, insomnia, and social isolation. A 2024 study found that 40% of CSU patients score above 10 on the Dermatology Life Quality Index - meaning their condition severely impacts every part of life. Treatment isn’t just about stopping hives. It’s about getting your life back. That’s why the right second-line treatment matters so much. The goal isn’t “a little better.” It’s “no hives, no swelling, no itching, no fear.” The tools to get there are finally here. The question is: are you ready to ask for them?How long does it take for second-line treatments to work?
Most second-line treatments take 4 to 12 weeks to show full effect. Omalizumab often starts helping within 4 weeks, but full benefit can take 3 months. Remibrutinib and dupilumab typically show noticeable improvement by week 8, with peak results around week 24. Don’t stop treatment too early - give it time.
Is omalizumab better than remibrutinib?
It depends. Omalizumab works well for IgE-driven CSU, but fails in about 30% of cases - especially those with IgG autoantibodies. Remibrutinib targets both IgE and IgG pathways and is taken orally, which improves adherence. Early data suggests remibrutinib may be more effective for autoimmune CSU, but omalizumab has longer-term safety data. If remibrutinib becomes available, it’s likely to become first choice for many patients.
Can I take remibrutinib if I’m on other medications?
Remibrutinib is generally safe with most medications, but it can interact with strong CYP3A4 inhibitors like ketoconazole or grapefruit juice. Always tell your doctor about every pill, supplement, or herb you take. Blood tests will be needed to check liver function, especially early in treatment.
What if I can’t afford omalizumab or don’t have insurance?
Omalizumab is expensive - often $1,500-$3,000 per injection. Some manufacturers offer patient assistance programs. Cyclosporine is much cheaper but requires close monitoring. Ask your doctor about clinical trials for remibrutinib or dupilumab - many are recruiting and provide free treatment and monitoring. Don’t give up - help is out there.
Do I need to stop antihistamines before starting second-line treatment?
No. Most guidelines recommend continuing antihistamines while starting second-line treatment. They can help manage symptoms during the transition. Some doctors may taper them slowly once the new drug starts working, but abruptly stopping can cause rebound flares.
Are there natural or alternative treatments that work for CSU?
No proven alternatives exist. Supplements like quercetin, vitamin D, or histamine-lowering diets may help a few people, but they don’t replace medical treatment. CSU is an immune disorder - not a diet issue. Relying on unproven methods can delay effective care and lead to worse outcomes. Always discuss supplements with your doctor.
Can CSU go away on its own without treatment?
Yes - but not for most. About 30-50% of people see symptoms resolve within 1-5 years without treatment. But for the rest, it can last decades. Waiting it out means enduring constant discomfort, sleep loss, and emotional strain. Second-line treatments don’t just control symptoms - they restore quality of life. Why wait when effective options exist?


Pooja Kumari
Okay so I’ve had CSU for 7 years and let me tell you, it’s not just the hives-it’s the sleepless nights, the panic when your lip swells before a meeting, the way your friends slowly stop asking you out because you ‘always cancel.’ I tried everything: antihistamines, steroids, even that weird elimination diet that made me cry over kale. Omalizumab helped a little, but I still had flares. Then I got on dupilumab off-label-my dermatologist was skeptical, but I begged. After 10 weeks? No hives. No itching. I cried in the shower. I’m not saying it’s perfect-I still get stressed and flare-but I can hold my kid again without worrying I’ll look like a monster. If you’re still on antihistamines and it’s not working? Please, just ask for more. You deserve better than this.
Also, the part about IgG vs IgE? Mind blown. I never knew my body was attacking itself. I thought I was just ‘allergic to life.’
Kiruthiga Udayakumar
Ugh. I hate when people treat CSU like it’s just ‘bad allergies.’ It’s not. It’s autoimmune. And if you’re still telling patients to ‘just take more Zyrtec’ you’re part of the problem. I’ve seen doctors dismiss this as ‘stress-related’ and then wonder why their patients end up in therapy. You don’t get to call it psychosomatic when your throat closes up. Remibrutinib being oral? Finally. Someone’s listening. And yes, dupilumab works-my sister’s been on it for 8 months and hasn’t had a flare since. Stop treating us like we’re dramatic. We’re just tired of being ignored.
Phil Kemling
It’s fascinating how medicine is finally catching up to the reality of chronic illness. For decades, we treated symptoms like they were the disease. Antihistamines suppress histamine, sure-but if the immune system is the arsonist, you can’t stop the fire by mopping up smoke. Omalizumab targets one kind of arsonist. Remibrutinib? It goes after the whole gang. And dupilumab? It’s like turning off the gas line. We’re moving from palliation to intervention. The real tragedy isn’t the disease-it’s the decades we wasted pretending it was just ‘bad skin.’ We’re finally asking: why? And that’s where healing begins.
Diana Stoyanova
Y’ALL. I just got my first remibrutinib prescription (early access program, yay!) and I’m literally screaming into my pillow. NO MORE INJECTIONS. JUST A LITTLE WHITE PILLS IN THE MORNING WITH MY COFFEE. I’ve been on omalizumab for 2 years. I love it, but I’m tired of being the lady who shows up to brunch with a syringe. And now? I can sleep through the night. I went to a concert last weekend. No hives. No anxiety. Just me, dancing like a weirdo. Also, I started keeping a symptom diary and turns out my flares spike every time I eat soy or get less than 6 hours of sleep. Who knew? Anyway-YOU ARE NOT ALONE. There’s hope. And it comes in pill form now. 🥳💊
Jenci Spradlin
so i had csu for 4 yrs and tried everything. omalizumab? worked for a bit then stopped. cyclosporine? made me feel like my kidneys were gonna explode. then i got on dupilumab off label and my life changed. no more hives, no more panic attacks before i leave the house. the only thing? insurance said no so i had to pay $1200 a month outta pocket. i work two jobs now just to afford it. but i’d do it again. if you’re on antihistamines and still flaring? stop. ask for more. your doctor might not know about remibrutinib yet-tell em to look it up. and pls, if you’re a doc reading this-stop being lazy. this isn’t just ‘itchy skin.’
Maggie Noe
I’m so tired of people saying ‘just take Benadryl’ like it’s a spa day 😤 I’ve had CSU since I was 19. Now I’m 32. I’ve missed weddings. I’ve missed my nephew’s first steps. I’ve cried in parking lots because I looked like I got into a fight with a nettle bush. Omalizumab gave me 60% relief. Dupilumab? 95%. And I didn’t even know it was an option until my dermatologist said ‘you’re not crazy, this is real.’
Remibrutinib? I’m already on the waiting list. Pill? Yes. Please. I hate needles. Also, the autoimmune angle? YES. I tested positive for IgG. That’s why nothing else worked. I’m not allergic to peanuts. I’m allergic to MYSELF. That’s wild. And terrifying. But now? I have tools. I have hope. And I’m not gonna shut up about it anymore. 🙌
Gregory Clayton
Why is America letting Big Pharma charge $3000 a shot for a drug that’s been around since 2014? I’m sick of this. We’re talking about people who can’t sleep, can’t work, can’t live-just because they’re broke or uninsured. And now they’re gonna charge even more for remibrutinib? It’s a pill, for god’s sake. Why not make it affordable? This isn’t medicine. It’s extortion. And don’t tell me ‘it’s research costs’-I’ve seen the profit margins. If I had to choose between my health and my rent? I’d choose rent. And that’s not a choice. It’s a crime.
Catherine Scutt
Let’s be real: if you’re still on antihistamines after 6 weeks, you’re wasting time. I’ve seen too many people cling to Zyrtec like it’s a religious artifact. It’s not. It’s a band-aid on a broken leg. And don’t get me started on ‘natural remedies.’ Quercetin? Vitamin D? Cute. But if your immune system is attacking your skin, you don’t need a smoothie-you need a biologic. If your doctor hasn’t mentioned omalizumab or dupilumab by now? Find a new one. This isn’t complicated. It’s immunology. Stop letting your pride keep you in pain.
Darren McGuff
As a dermatologist who’s treated CSU for 18 years, I’ve watched this field evolve from ‘try more antihistamines’ to precision immunology. The shift from IgE-only thinking to recognizing IgG-mediated disease has been revolutionary. Remibrutinib’s oral delivery is a game-changer-adherence rates jump when you don’t need to inject yourself monthly. But let’s not oversell it: we still need long-term safety data. And dupilumab? Brilliant, but insurance denial is a nightmare. My advice? Get tested for autoantibodies. Know your subtype. And if cyclosporine is your last resort? Do it under strict monitoring. You’re not failing-you’re fighting a complex disease. And you’re not alone.
Ashley Kronenwetter
Thank you for writing this with such clarity and compassion. I’ve spent years trying to explain to my family that this isn’t ‘just a rash.’ The emotional toll is as real as the physical one. I’m grateful for the progress in treatment, but I’m also saddened by how long it took. I hope this post reaches those still suffering in silence. You are not broken. You are not imagining it. And there are treatments that can restore your life. Please, advocate for yourself. Your health matters.
Johanna Baxter
I’ve been on 3 different biologics and none of them worked until I got on cyclosporine. I know it’s scary. I know the side effects. But I had hives for 5 years straight. I couldn’t wear a shirt without itching through it. I lost my job. My marriage. I didn’t care anymore. Then my doctor said ‘this might kill you slowly, but it’ll let you live now.’ So I took it. I’ve been on it for 8 months. No hives. I’m alive. And yes, my kidneys are being watched. But I’d rather have monitored kidneys than a funeral. If you’re scared? I get it. But don’t let fear keep you in hell.
Jerian Lewis
People need to stop romanticizing ‘natural healing.’ CSU isn’t caused by gluten or stress or bad vibes. It’s an autoimmune disorder. If you’re telling someone to ‘meditate it away’ or ‘drink turmeric tea,’ you’re not helping. You’re delaying real care. I watched my sister die because she trusted a ‘healer’ instead of a doctor. Don’t be her. Ask for the science. Demand the tests. Take the pill. The drugs work. Stop letting pseudoscience win.
Patty Walters
just wanted to say-i’m a nurse who’s had csu since i was 21. i’ve been on everything. omalizumab? worked for 14 months. dupilumab? miracle. remibrutinib? waiting for approval. the thing no one says? it’s not about the hives anymore. it’s about the fear. the constant dread that the next itch means swelling. that the next meal means an epipen. that the next hug might be the last one before you’re in the er. i started a support group. 37 women. all of us have been told we’re ‘overreacting.’ we’re not. we’re survivors. if you’re reading this and still on antihistamines? please. just ask for more. we’ve been waiting too long.
tali murah
Oh, so now we’re supposed to believe remibrutinib is the ‘miracle pill’ because a phase 3 trial showed 30% complete response? Let’s not forget: 70% still had symptoms. And dupilumab? Still off-label. And cyclosporine? Kidney damage. This isn’t progress-it’s repackaged desperation. The pharmaceutical industry thrives on chronic illness. They don’t want to cure it. They want you to keep paying. And you? You’re the product. Enjoy your little pill. I’ll be over here, quietly dying with my dignity intact.