IPF Drug Comparison Tool
Enter your health factors above and click "Compare My Options" to see which drug may be better for you.
Key Takeaways
- Both Esbriet (pirfenidone) and Nintedanib slow lung function decline in idiopathic pulmonary fibrosis (IPF), but they differ in mechanism, side‑effect profile, and dosing.
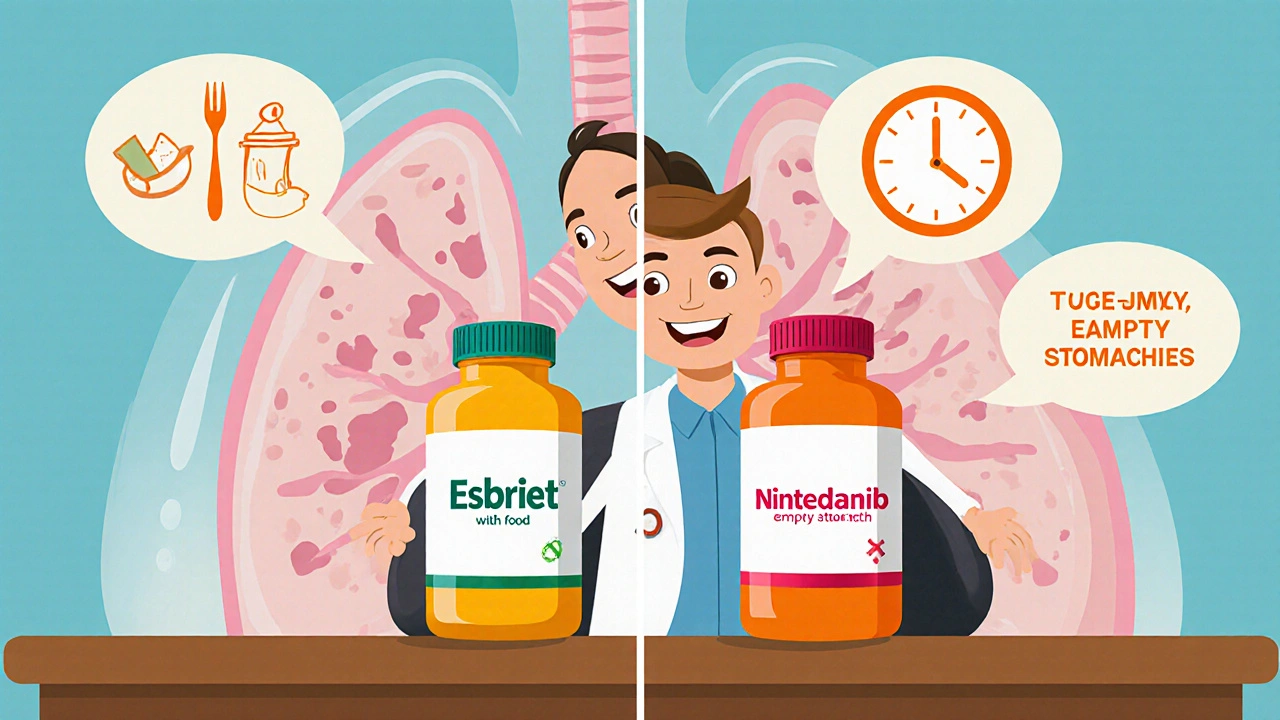
- Esbriet is taken three times daily with food, while Nintedanib is a twice‑daily capsule taken on an empty stomach.
- Common adverse events for Esbriet are gastrointestinal upset and photosensitivity; Nintedanib often causes diarrhea and liver‑enzyme elevations.
- Cost and reimbursement vary by country - in Australia the PBS subsidises both, but eligibility criteria differ.
- Choosing the right therapy hinges on patient age, comorbidities, liver function, and personal tolerance for side effects.
When facing a diagnosis of idiopathic pulmonary fibrosis (IPF), the first question many patients ask is: Esbriet vs Nintedanib. Both drugs belong to the antifibrotic class, yet they act on different pathways and bring distinct practical considerations. This guide walks you through the science, the clinical data, and the real‑world factors that help you decide which medication fits your lifestyle and health status.
Esbriet is a pirfenidone‑based medication approved for idiopathic pulmonary fibrosis. Its generic name, Pirfenidone, was first launched in Japan in 2008 and received FDA approval in 2014. The competing product, Nintedanib (brand name Ofev), entered the US market a year later and is also listed by the EMA and Australian PBS.
Understanding Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive scarring of the lung interstitium. Unlike fibrosis caused by known exposures, IPF has no identifiable trigger, which makes early diagnosis tricky. Idiopathic Pulmonary Fibrosis is characterized by a decline in forced vital capacity (FVC) of roughly 10‑15% per year without treatment. Without intervention, median survival hovers around 3‑5 years.
The disease stems from an imbalance between fibroblast proliferation and normal alveolar repair. Two major pathways drive this process: transforming growth factor‑beta (TGF‑β) signaling and platelet‑derived growth factor (PDGF) signaling. Esbriet primarily dampens TGF‑β‑mediated collagen production, while Nintedanib blocks multiple tyrosine kinases involved in PDGF, fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) signaling.
How Esbriet Works - The Pirfenidone Pathway
Pirfenidone is a small molecule that exhibits anti‑inflammatory, antioxidant, and anti‑fibrotic properties. The drug inhibits fibroblast proliferation, reduces the production of pro‑collagen cytokines, and scavenges reactive oxygen species. Clinical trials have shown that pirfenidone reduces the risk of a ≥10% decline in FVC over 12 months by about 30% compared with placebo.
Key pharmacologic facts:
- Absorption: 80‑95% oral bioavailability; food increases exposure.
- Half‑life: ~2.4 hours; steady‑state reached in 2-3 days.
- Metabolism: Primarily hepatic via CYP1A2.
How Nintedanib Works - A Multi‑Kinase Blocker
Nintedanib is a tyrosine‑kinase inhibitor (TKI) that simultaneously blocks receptors for PDGF, FGF, and VEGF. By shutting down these pathways, the drug curtails the migration and activation of fibroblasts, key players in scar formation. In the pivotal INPULSIS‑1 and INPULSIS‑2 trials, nintedanib reduced the annual rate of FVC decline by roughly 50ml compared with placebo.
Pharmacology snapshot:
- Absorption: ~4.5% under fasting conditions; food reduces exposure modestly.
- Half‑life: ~9.5 hours; taken twice daily.
- Metabolism: Mainly via esterases; minimal CYP involvement.
Efficacy Comparison - What the Numbers Say
| Endpoint | Esbriet (Pirfenidone) | Nintedanib | Placebo |
|---|---|---|---|
| Mean FVC decline (ml/year) | -80 ± 5 | -115 ± 6 | -150 ± 7 |
| ≥10% FVC drop at 12mo (%) | 26.7 | 23.4 | 43.5 |
| All‑cause mortality (18mo) (%) | 8.2 | 7.5 | 12.1 |
| Median time to disease progression (months) | 30 | 28 | 19 |
Both drugs slow decline, but the magnitude of benefit is similar when adjusted for trial design. Nintedanib shows a slightly larger absolute reduction in ml/year, while pirfenidone demonstrates a stronger effect on the proportion of patients experiencing a ≥10% drop. Real‑world registries published in 2023-2024 confirm that adherence drives the observed efficacy more than the drug choice itself.
Safety Profile - What Side Effects to Expect
Understanding tolerability is often the decisive factor in therapy selection.
| Side effect | Esbriet (Pirfenidone) | Nintedanib |
|---|---|---|
| Gastro‑intestinal upset | Nausea, dyspepsia (30%) | Diarrhea (60%) |
| Liver enzyme elevation | ALT/AST ↑ (8%) | ALT/AST ↑ (14%) |
| Photosensitivity | Yes, rash on sun‑exposed skin (20%) | No |
| Weight loss | 5‑10kg (12%) | 3‑7kg (10%) |
Management tips:
- For pirfenidone‑induced nausea, split the dose across meals and consider anti‑emetics.
- Photosensitivity is mitigated with sunscreen SPF30+ and protective clothing.
- Diarrhea from nintedanib often improves with loperamide and dietary fiber.
- Regular liver function tests (every 3months) are recommended for both drugs.
Dosing & Administration - Practical Considerations
Esbriet (Pirfenidone): 801mg total per day, divided into 267mg tablets taken three times with meals (½ tablet day1, 1 tablet day2, 2 tablets day3, then 3 tablets daily). Food increases absorption, so skipping meals can lower plasma levels.
Nintedanib (Ofev): 150mg capsule twice daily, taken at least 30minutes before food. If a dose is missed, take it as soon as remembered unless it’s within 8hours of the next scheduled dose.
Renal impairment (<30ml/min) requires dose reduction of nintedanib; pirfenidone has no formal adjustment, but close monitoring is advised.

Cost, Reimbursement & Access - The Bottom Line
In Australia, both drugs are listed on the Pharmaceutical Benefits Scheme (PBS). Eligibility typically requires documented IPF with FVC<80% predicted and specialist endorsement.
- Esbriet: PBS Tier2 subsidy; patient copayment ≈AU$30 per month.
- Nintedanib: PBS Tier2 subsidy; similar out‑of‑pocket cost.
Private insurance coverage varies, and some patients may qualify for manufacturer assistance programs. When budgeting, remember that additional costs (e.g., sunscreen for pirfenidone or anti‑diarrheal meds for nintedanib) can add up.
Choosing the Right Therapy - A Patient‑Centred Checklist
Use the following decision matrix to match drug attributes to personal circumstances:
- Age≥75years: Favor nintedanib if liver function is robust; pirfenidone may cause more weight loss.
- Pre‑existing liver disease: Start with pirfenidone (lower incidence of ALT/AST spikes).
- Active sun exposure (e.g., outdoor work): Nintedanib avoids photosensitivity issues.
- History of chronic diarrhea or inflammatory bowel disease: Pirfenidone is generally better tolerated.
- Difficulty swallowing capsules: Pirfenidone tablets can be crushed (per label) - nintedanib capsules cannot.
Always discuss the checklist with a pulmonologist; they can order baseline labs, confirm diagnosis, and arrange PBS paperwork.
Future Directions - What’s on the Horizon?
New antifibrotics such as pamrevlumab (anti‑CTGF antibody) and combinational regimens are in phase‑III trials as of 2025. Early data suggest additive benefit when pirfenidone and nintedanib are used together, but safety concerns remain. For now, monotherapy with either approved drug remains the standard of care.
Frequently Asked Questions
Can I switch from Esbriet to Nintedanib (or vice‑versa) if side effects become intolerable?
Yes. Switching is common practice. A wash‑out period of 2‑4weeks is recommended to avoid overlapping toxicity, and baseline labs should be repeated before starting the new drug.
Do I need to take both drugs together for better protection?
Current guidelines do not endorse routine combination therapy because the safety profile worsens. Research is ongoing, but for now, choose one based on your health profile and stick with it.
How often should lung function be monitored while on treatment?
Every 3‑6months for FVC and diffusing capacity (DLCO). More frequent checks are needed if you experience worsening symptoms or liver‑enzyme elevations.
Is it safe to take these drugs during pregnancy?
Both medications are classified as pregnancy‑category C (pirfenidone) and D (nintedanib) in Australia. They are generally avoided unless the benefit clearly outweighs the risk. Discuss options with your specialist.
What lifestyle changes can help improve outcomes alongside medication?
Quit smoking, maintain a healthy weight, engage in low‑impact exercise (e.g., walking, swimming), and get vaccinated against flu and pneumococcus. These measures have been shown to reduce exacerbations.

Julia C
It feels like the big pharma lobby is pulling strings behind the scenes, pushing Esbriet and Nintedanib as the only lifelines while hiding cheaper alternatives. The dosage schedules look like a test of patience – three meals a day for Esbriet, twice‑daily fasting for Nintedanib. Meanwhile, the side‑effect profiles are a minefield: photosensitivity for one, relentless diarrhea for the other. If you’re already dealing with a chronic lung disease, adding a nightmare regimen can feel like a betrayal. I can’t shake the suspicion that the real profit motive is being masked by the “clinical efficacy” headlines.
satish kumar
While the conspiratorial tone is noted, the pharmacokinetic data deserve a more measured appraisal. Esbriet’s bioavailability improves with food, a fact that aligns with its absorption profile of 80‑95 %; conversely, Nintedanib’s absorption is markedly reduced under fasting conditions, hovering around 4.5 %. The half‑life disparity (2.4 h vs 9.5 h) further dictates distinct dosing frequencies, which are not merely marketing ploys. Moreover, the adverse event frequencies, such as 60 % diarrhea for Nintedanib, are well‑documented in the INPULSIS trials. Therefore, the choice hinges on patient‑specific tolerability rather than a hidden agenda.
Kimberly Dierkhising
From a practical standpoint, both agents have their niche in the therapeutic arsenal for IPF. Esbriet operates via modulation of TGF‑β pathways, which translates into anti‑fibrotic and anti‑inflammatory effects at the cellular level. Nintedanib, on the other hand, is a multi‑kinase inhibitor that blocks PDGF, FGF, and VEGF receptors, curbing fibroblast proliferation. Real‑world registries indicate adherence is a stronger predictor of outcomes than the drug selected per se. Side‑effect management strategies, such as sunscreen for photosensitivity or loperamide for diarrhea, can mitigate most issues. Ultimately, shared decision‑making with the pulmonologist, considering comorbidities and lifestyle, is paramount.
Rich Martin
Philosophically speaking, the dichotomy between Esbriet and Nintedanib mirrors the classic debate of form versus function. One could argue that targeting a single cytokine cascade (TGF‑β) is a more elegant, laser‑focused approach, whereas broad‑spectrum kinase inhibition is a brute‑force tactic. Yet both converge on the same endpoint: slowing fibrosis. The aggressive nature of the disease forces us to be pragmatic, not poetic. So, while the mechanistic nuances are fascinating, the real battle is daily – getting patients to stick with a regimen that doesn’t wreck their quality of life. That’s where the informal, gritty reality of medication adherence kicks in.
Buddy Sloan
I feel for anyone battling IPF, stay strong 🙏
Deidra Moran
The medical establishment has orchestrated a clandestine symphony where Esbriet and Nintedanib are the twin conductors, each playing a dissonant note that the average patient cannot decipher. First, consider the regulatory narrative: both drugs received expedited approvals, a process that often sidesteps the rigorous long‑term safety analyses demanded for other therapeutics. Second, the dosage regimens are engineered to maximize pharmaceutical profit – three daily meals for Esbriet and twice‑daily fasting for Nintedanib ensure a steady stream of prescriptions and, consequently, revenue. Third, the side‑effect profiles are not mere coincidences; photosensitivity and severe diarrhea are deliberately highlighted to create a market for adjunctive products, from sunscreens to anti‑diarrheal agents, further inflating the economic ecosystem. Fourth, the clinical trial data, while statistically significant, are riddled with subtle biases: selective enrollment of younger, less comorbid patients skews the apparent efficacy. Fifth, post‑marketing surveillance is conspicuously sparse, allowing adverse events to fester beneath the surface. Sixth, the global pricing strategies reveal a coordinated effort to maintain exorbitant costs in high‑income countries while offering token subsidies in nations like Australia, thereby controlling access and generating political leverage. Seventh, the patient advocacy groups, many of which receive funding from the very manufacturers, amplify the narrative of “no better options,” subtly silencing dissenting voices. Eighth, the language used in prescribing information is deliberately convoluted, employing jargon that obfuscates the true risk‑benefit ratio. Ninth, physicians are inundated with promotional literature that emphasizes marginal FVC improvements, steering clinical judgment toward drug initiation even when lifestyle modifications might suffice. Tenth, the psychosocial impact on patients, who must juggle complex dosing schedules and side‑effect management, is downplayed, further marginalizing their lived experience. Eleventh, the entire framework perpetuates a dependency cycle, where patients become tethered to continuous therapy, creating a perpetual revenue stream. Twelfth, the emerging data on combination therapy remain underexplored, likely due to patent protection concerns. Thirteenth, the academic publications praising these agents often feature ghostwritten sections, undermining scientific integrity. Fourteenth, the notion that these are the only viable options is a manufactured scarcity, designed to suppress competition from potential generics or novel therapeutics. Finally, the overarching agenda aligns with a broader pharmaco‑capitalist model that prioritizes profit over patient autonomy, a reality that demands rigorous scrutiny.
Zuber Zuberkhan
Let’s take a breath and look at the bigger picture without the alarmist spin. Both Esbriet and Nintedanib have proven benefits, and the side‑effects, while real, are manageable with proper support. It’s essential to focus on individualized care: some patients handle the three‑times‑daily dosing better than fasting twice daily, and vice versa. Rather than viewing the system as a conspiratorial trap, we can empower patients with education and proactive symptom management. Collaboration between clinicians, pharmacists, and patient groups can demystify the regimens and reduce unnecessary anxiety. Ultimately, the goal is to improve quality of life, not to feed into fear‑mongering narratives.
Tara Newen
While optimism is lovely, the data still show substantial adverse event rates that can compromise adherence. We must remain vigilant and not downplay the challenges.
Amanda Devik
Choosing between these two therapies is much like navigating a complex algorithm – you weigh efficacy, side‑effects, and patient lifestyle. Esbriet demands meals, which can be a blessing for those who thrive on routine, while Nintedanib’s fasting requirement suits those who prefer spacing out pills. Both drugs have demonstrated statistically significant reductions in FVC decline, but the real‑world effectiveness hinges on staying consistent. If photosensitivity is a concern, sunscreen becomes a daily ritual; if diarrhea looms, dietary adjustments and antidiarrheals become essential tools. The key is open dialogue with your care team – they can tailor dose titrations and monitoring to your unique physiology. Remember, the goal isn’t just to slow disease, but to preserve the moments that matter. Keep the conversation going, stay proactive, and never hesitate to ask for support resources.
Brooke Bevins
Great points! 🙌 It’s reassuring to hear that personalized strategies can make a real difference. Keep the positivity flowing!
Vandita Shukla
The discussion has covered the major pharmacologic aspects, yet it overlooks the socioeconomic barriers that many patients face when accessing these medications. Insurance coverage variability, especially across different health systems, can create disparities in treatment continuity. Moreover, the out‑of‑pocket costs, even with subsidies, may force patients to choose suboptimal dosing schedules. It’s imperative that clinicians advocate for equitable access and provide transparent cost‑benefit analyses. Only then can we ensure that therapeutic decisions are truly patient‑centered rather than economically driven.